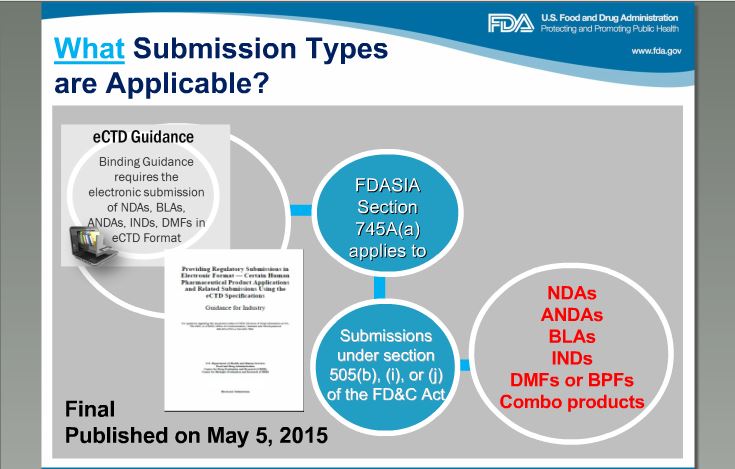
Please note this date:
DMF is just one of the requirement
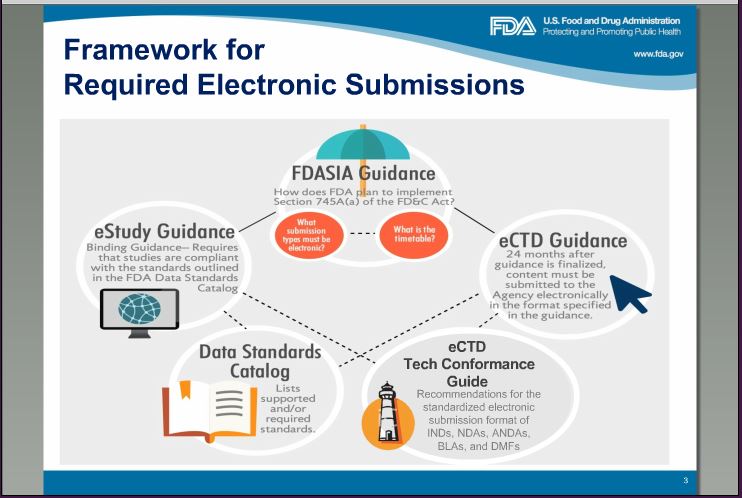
FDA Framework for Electronic Submission:
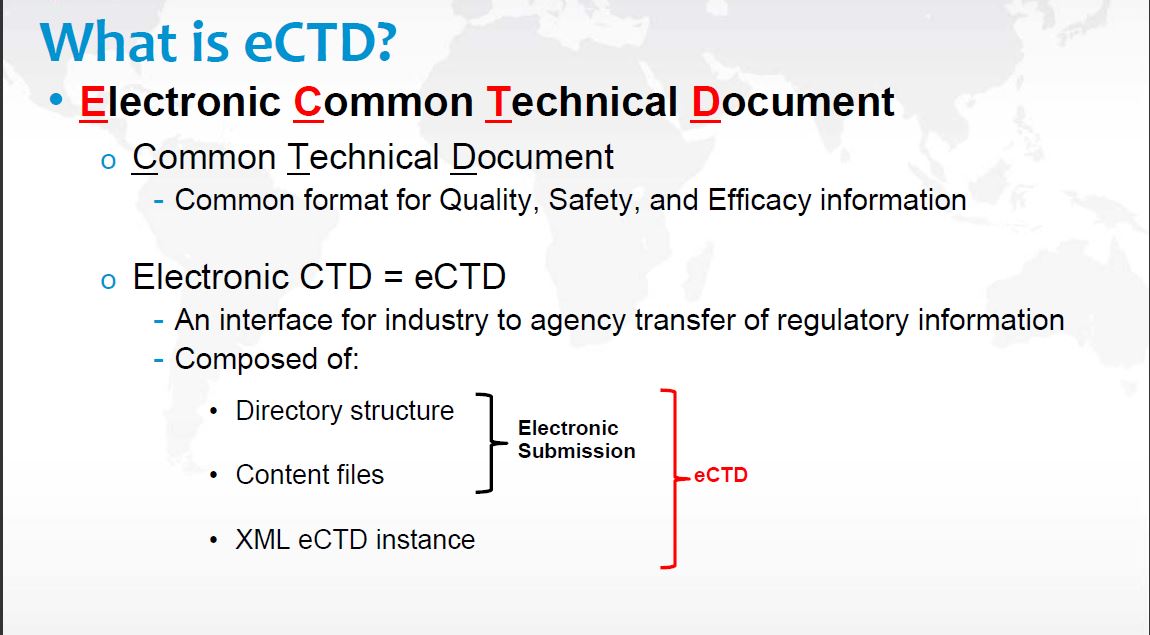
Then what is eCTD
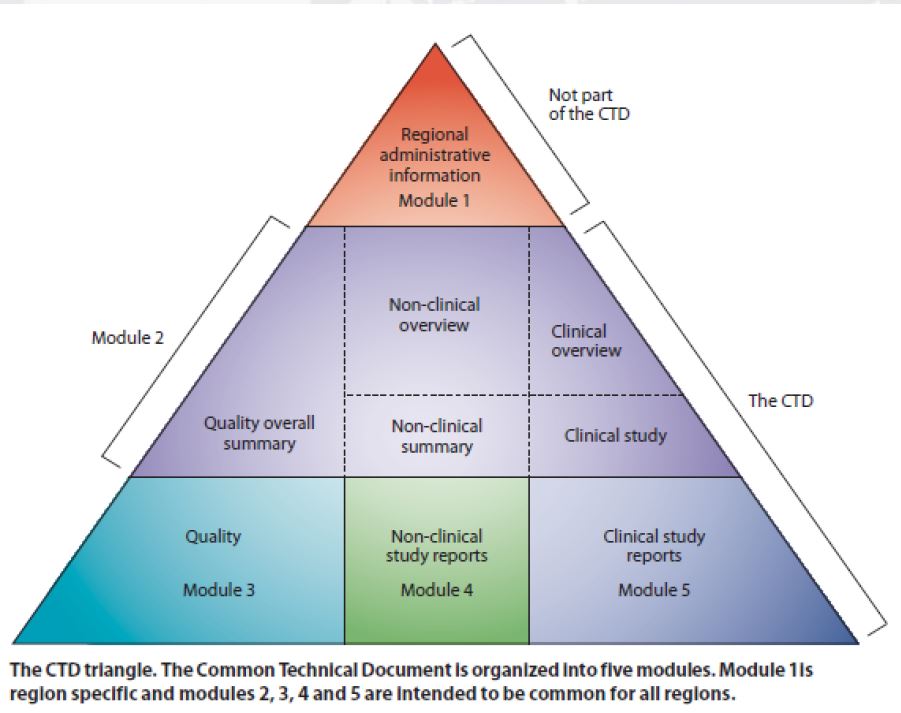
How about CTD:
We specialize eCTD update or new submission. If you need more information, please contact us at jsc@fdaconsultantnusagent.com.